Abstract
Introduction:
Altered erythrocyte physiology in Sickle Cell Disease (SCD) results in severe vascular complications, including vaso-occlusion (VOC) along with various other manifestations, such as thrombosis and end organ damage. Moreover, platelet activation endothelial dysfunction and fibrin formation, are critical processes implicated in thrombo-inflammatory vasculopathy in SCD. Since flavonoids intake is associated with lowered adverse cardiovascular disease outcomes and cancer associated thrombosis, we are evaluating the effects of the flavonoid Isoquercetin (IQ) on thrombo-inflammatory biomarkers in patients with SCD (NCT:04514510). Here we report the mechanistic effects of Quercetin (Que), an aglycone that differs from IQ by the absence of 3-linked glucoside moiety, on markers of erythrocyte and platelet activation in patients with SCD.
Methods:
We studied patients with SCD (Genotype SS, mean age: 39 yrs, N=8) and ethnic matched controls (Genotype AA, mean age: 42 yrs, N=8). Erythrocyte reactive oxygen species (ROS) was measured in washed red cells by flow cytometry using the fluorescent probe 2', 7'-dichlorofluorescein diacetate (DCFDA) in the presence or absence of Quercetin (Que) (100 µM). Effects of in vitro erythrocyte ROS induction by hydrogen peroxide (H 2O 2) and ROS inhibition by N-acetyl-L-cysteine (NAC) were evaluated. Baseline and agonist-induced (ADP 20 µM) platelet P-selectin expression in vitro in the presence or absence of Que (100 µM) was assessed by flow cytometry.
Results:
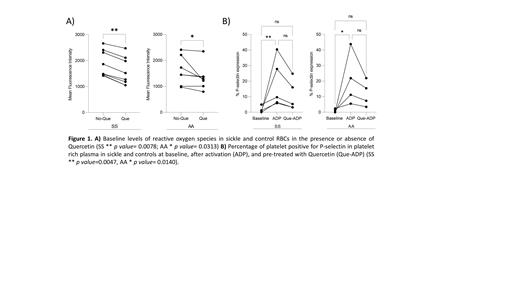
At baseline, SS erythrocytes demonstrated elevated levels of ROS reflected by greater florescent intensity with DCFDA when compared with erythrocytes from ethnic matched controls (SS mean= 1884, SD: 508.8 vs. AA mean: 1604, SD: 554.5; p value= 0.3798). Stimulation of AA erythrocytes in vitro with H 2O 2 led to accumulation of ROS that approached levels similar to those exhibited by SS erythrocytes at baseline (AA mean: 1890, SD: 748.3). Pre-treatment of SS and AA erythrocytes in vitro with the antioxidant NAC exhibited minimal effects on ROS in SS erythrocytes (SS baseline+NAC mean: 1942, SD: 526.8, p value 0.4609 and SS H 2O 2+NAC mean: 1889, SD: 437.4, p value 0.5469) but lowered ROS levels in both baseline and H 2O 2 treated AA erythrocytes (AA baseline+NAC mean: 1442, SD: 453.9, p value 0.1563 and AA H 2O 2+NAC mean: 1581, SD: 518.7, p value 0.078). Pre-treatment of SS and AA erythrocytes in vitro with Que substantially lowered erythrocyte ROS in patients and controls at the baseline (SS baseline ROS with Que mean: 1582, SD: 542.0, p value 0.0078; AA baseline ROS with Que mean: 1341, SD: 490.9, p value 0.0313; Fig. 1A), and after H 2O 2 stimulation (SS H 2O 2 induced ROS+Que mean: 1677, SD: 572.5, p value 0.0156; AA H 2O 2 induced ROS+Que mean: 1417, SD: 512, p value 0.0078). Pre-treatment of erythrocytes with a combination of Que and NAC did not reveal synergistic anti-oxidant activity (SS NAC+Que mean: 1616, SD: 464.8; AA NAC+Que mean: 1333, SD: 524.1).
We simultaneously investigated the effects of Que on platelet P-selectin expression in the same subjects. As expected, baseline platelet surface P-selectin expression was higher in SS patients compared with controls (SS N=5, mean: 1.48%, Max: 4.9%, Min: 0.03%; AA N=4, mean: 0.89%, Max: 2.3%, Min: 0%; p value 0.55). Stimulation of platelet rich plasma (PRP) with ADP increased surface P-selectin expression in comparison with baseline, in both patients and controls (SS N=5, mean: 18.04%, Max: 40.4%, Min: 5.8%, p value 0.0047; AA N=4, mean: 20.60%, Max: 43.8%, Min: 5.4%; p value 0.0140). Pre-treatment of PRP with Que prior to ADP stimulation revealed a trend toward decreased platelet surface P-selectin expression in both patients and controls (SS N=5, mean: 10.49%, Max: 24.8%, Min: 3.1%; AA N=4, mean: 12.09%, Max: 21.9%, Min: 3.4%; Fig 1B) although this did not return to baseline.
Conclusion:
The flavonoid Quercetin appears to have a beneficial effect in lowering erythrocyte ROS and platelet surface P-selectin and warrant further evaluation as a strategy to ameliorate thrombo-inflammatory pathophysiology in sickle hemoglobinopathies.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal